|
|
Projects in This Lab
Control of Notch signaling by endocytosis
Notch signaling is required universally for development in every tissue in all metazoans, and defects in the mechanism are associated with many human developmental diseases and cancer. A peculiar feature of the Notch pathway is the extent to which its activation and regulation depend on endocytosis. A mysterious feature of Notch signaling is that ligand must be internalized by the signaling cells in order for them to activate the Notch receptor on adjacent cells. We are trying to understand why ligand endocytosis is necessary for signaling. In addition, we are exploring how regulation of endocytic factors contributes to regulation of signaling. We have shown that two endocytic proteins, Epsin (aka Liquid facets, or Lqf) and Auxilin, are absolutely necessary for ligand internalization and signaling. We are investigating how Epsin and Auxilin function in the signaling cells, and how they are regulated.
The Notch signaling pathway.
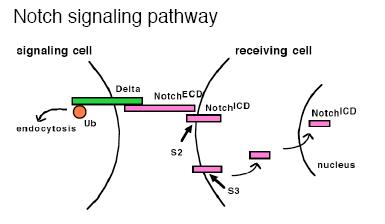 The Notch receptor is a singlepass transmembrane protein that in Drosophila has two different transmembrane ligands, Delta and Serrate. There are many unusual features to the mechanism of Notch activation. One is that part of the receptor protein itself acts as a transcription factor. Ligand binding activates cleavage of the Notch extracellular domain (S2 cleavage) by an ADAM protease, which is then followed by cleavage of the intracellular domain (S3 cleavage) by the a-secretase complex. The released intracellular domain enters the nucleus, where, along with Suppressor of Hairless [Su(H)], it regulates target gene expression. Second, Notch signaling is regulated by endocytosis in a variety of ways in different cellular contexts. Most remarkably, the ligand must undergo endocytosis by the signaling cells in order to signal.
The Notch receptor is a singlepass transmembrane protein that in Drosophila has two different transmembrane ligands, Delta and Serrate. There are many unusual features to the mechanism of Notch activation. One is that part of the receptor protein itself acts as a transcription factor. Ligand binding activates cleavage of the Notch extracellular domain (S2 cleavage) by an ADAM protease, which is then followed by cleavage of the intracellular domain (S3 cleavage) by the a-secretase complex. The released intracellular domain enters the nucleus, where, along with Suppressor of Hairless [Su(H)], it regulates target gene expression. Second, Notch signaling is regulated by endocytosis in a variety of ways in different cellular contexts. Most remarkably, the ligand must undergo endocytosis by the signaling cells in order to signal.
Endocytosis and the fates of endocytosed proteins.
Internalization of plasma membrane proteins occurs through clathrin-dependent and clathrin-independent mechanisms. In the clathrin pathway, with the help of the AP-2 adapter complex, clathrin triskelia form a cage structure around invaginated membrane, and the activity of the GTPase Dynamin (Shibire in Drosophila) results in vesicle scission. Newly formed endosomes fuse with the sorting endosome, and the internalized proteins either are shunted to the recycling endosome and sent back to the plasma membrane, or targeted to the multivesicular body (MVB)/late endosome prior to secretion as an exosome, or Notch signaling pathway lysosomal degradation. Clathrin-independent internalization occurs at sites of lipid rafts which are cholesterol-rich regions of the plasma membrane. The protein Caveolin mediates some of the clathrin-independent events in vertebrate cells (forming caveolae), and flotillin mediates others. Drosophila have no caveolin, but flotillin is present.
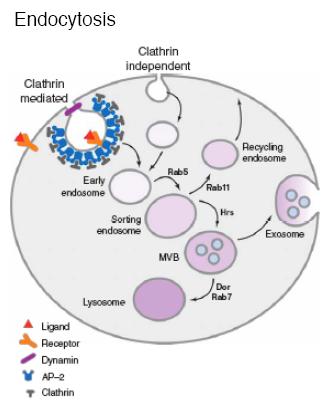 Regulation of endocytosis.
Several different kinds of proteins and lipids regulate internalization and endosomal sorting. Rab proteins are membrane associated, Ras-like GTPases that control membrane fusion. Different Rabs are associated with particular endosomes. Inositol phospholipids (phosphoinositides) constitute a small fraction of the phospholipids in the plasma membrane and endosomal membranes. Distinct regions of the plasma membrane and different endosomes are enriched in particular varieties of phosphoinositides which bind with different affinities to proteins with lipid-binding domains. For example, the ENTH domain of Epsin (see below) binds PI(4,5)P2, which is enriched at the plasma membrane in vertebrate cells. Some transmembrane proteins have cytoplasmically located internalization signals that are part of their primary amino acid sequence, and these may bind AP-2. Alternatively, a ubiquitin (Ub) polypeptide that serves as an endocytosis signal may be added posttranslationally to the cytoplasmic domain, and these signals may bind Epsin.
Regulation of endocytosis.
Several different kinds of proteins and lipids regulate internalization and endosomal sorting. Rab proteins are membrane associated, Ras-like GTPases that control membrane fusion. Different Rabs are associated with particular endosomes. Inositol phospholipids (phosphoinositides) constitute a small fraction of the phospholipids in the plasma membrane and endosomal membranes. Distinct regions of the plasma membrane and different endosomes are enriched in particular varieties of phosphoinositides which bind with different affinities to proteins with lipid-binding domains. For example, the ENTH domain of Epsin (see below) binds PI(4,5)P2, which is enriched at the plasma membrane in vertebrate cells. Some transmembrane proteins have cytoplasmically located internalization signals that are part of their primary amino acid sequence, and these may bind AP-2. Alternatively, a ubiquitin (Ub) polypeptide that serves as an endocytosis signal may be added posttranslationally to the cytoplasmic domain, and these signals may bind Epsin.
Signaling by Notch ligands requires their internalization by signaling cells.
This surprising observation has come from studies in cell culture and in vivo, mainly in wing and eye imaginal discs and in developing bristles. In many instances, Delta is observed in shibire+-dependent intracellular vesicles, and the results of many different experiments connect Delta and Serrate endocytosis with signaling. For example, mutant Delta proteins that cannot be internalized cannot signal. Later, it was discovered that either of two RING finger Ub-ligases, Neuralized or D-Mib (Drosophila Mindbomb), is required in the signaling cells for Delta or Serrate
 ubiquitination and internalization. Experiments with Drosophila Epsin (Liquid facets) and Auxilin have reinforced this idea.
ubiquitination and internalization. Experiments with Drosophila Epsin (Liquid facets) and Auxilin have reinforced this idea.
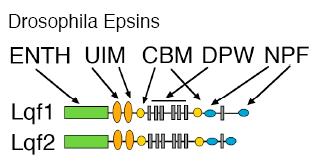
What is Epsin?
Epsin is a multi-modular protein, first identified in vertebrate cells, that functions in vesicle formation at the plasma membrane. Epsin has an ENTH (Epsin N-terminal homology) domain, which inserts into the membrane, clathrin binding motifs (CBMs), ubiquitin interaction motifs (UIMs), DPW motifs that bind AP-2, and NPF motifs that bind other endocytic accessory factors.
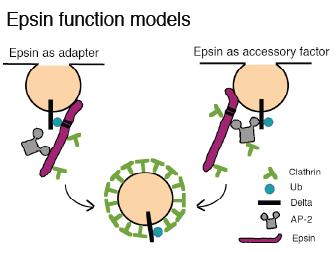 The model for endocytic Epsin function is controversial, but the simplest idea that fits with most of the experimental data regarding its role in Delta endocytosis is that Epsin binds the plasma membrane and induces its curvature with the ENTH domain, throughts UIMs recognizes ubiquitin (Ub) as an internalization signal, and brings clathrin, AP-2, and other endocytic factors to the membrane in order to internalize clathrin-coated vesicles (CCVs). Epsin may serve as an alternate, AP-2-independent adapter. Also, Epsin might in some contexts serve as an accessory factor for AP-2-mediated CCV formation. Epsin may also function in clathrin dep endent endocytosis.
The model for endocytic Epsin function is controversial, but the simplest idea that fits with most of the experimental data regarding its role in Delta endocytosis is that Epsin binds the plasma membrane and induces its curvature with the ENTH domain, throughts UIMs recognizes ubiquitin (Ub) as an internalization signal, and brings clathrin, AP-2, and other endocytic factors to the membrane in order to internalize clathrin-coated vesicles (CCVs). Epsin may serve as an alternate, AP-2-independent adapter. Also, Epsin might in some contexts serve as an accessory factor for AP-2-mediated CCV formation. Epsin may also function in clathrin dep endent endocytosis.
 What is Auxilin?
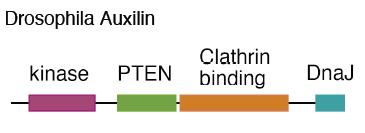
Drosophila Auxilin is also a multi-modular protein, most similar to vertebrate Auxilin2, or GAK (Cyclin G-associated kinase). Auxilin has N-terminal kinase and PTEN (phosphatase and tensin-related) domains, whose functions are unclear. We showed that neither of these Auxilin domains has any obvious essential function in Drosophila. The part of Auxilin required for Delta signaling is the C-terminus, which includes a Clathrin-binding domain and a J domain. Auxilin is an adapter for the ATPase Hsc70 (Heat shock cognate 70); Auxilin binds Hsc70 with its J domain and also binds Clathrin cages, which are Hsc70 substrates. Auxilin catalyzes the binding of Hsc70 to Clathrin cages and stimulates its ATPase activity. The evidence is most solid for two direct functions of Auxilin, one in internalization and a second in uncoating CCVs. At the internalization step, Hsc70/Auxilin exchanges Clathrin on cages forming at the plasma membrane, which may help to constrict vesicles prior to scission. After scission, Hsc70/Auxi lin removes the Clathrin cages, recycling Clathrin and allowing vesicle fusion with endosomes.
What is Auxilin?
Drosophila Auxilin is also a multi-modular protein, most similar to vertebrate Auxilin2, or GAK (Cyclin G-associated kinase). Auxilin has N-terminal kinase and PTEN (phosphatase and tensin-related) domains, whose functions are unclear. We showed that neither of these Auxilin domains has any obvious essential function in Drosophila. The part of Auxilin required for Delta signaling is the C-terminus, which includes a Clathrin-binding domain and a J domain. Auxilin is an adapter for the ATPase Hsc70 (Heat shock cognate 70); Auxilin binds Hsc70 with its J domain and also binds Clathrin cages, which are Hsc70 substrates. Auxilin catalyzes the binding of Hsc70 to Clathrin cages and stimulates its ATPase activity. The evidence is most solid for two direct functions of Auxilin, one in internalization and a second in uncoating CCVs. At the internalization step, Hsc70/Auxilin exchanges Clathrin on cages forming at the plasma membrane, which may help to constrict vesicles prior to scission. After scission, Hsc70/Auxi lin removes the Clathrin cages, recycling Clathrin and allowing vesicle fusion with endosomes.
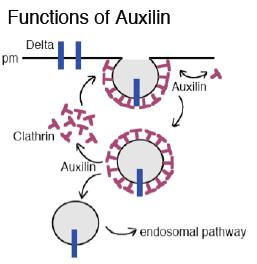
Epsin and Auxilin are essential for ligand internalization and signaling.
We showed that Epsin is required absolutely for Delta signaling in the eye disc. The results of genetic experiments in the wing disc led to the same conclusion. In addition, these experiments showed that Epsin-dependent endocytosis requires ligand mono-ubiquitination by Neuralized or D-Mib, which suggests that Epsin recognizes mono-Ub on the ligand extracellular domain. One of the most remarkable results of these experiments is that the lqf null phenotype is essentially identical to the Delta and/or Serrate null phenotypes. Other signaling pathways are functional in the absence of Epsin, and the cells do not die. Thus, although it may have other non-essential roles in imaginal discs, Epsin is required specifically for Notch ligand internalization. Even more remarkably, we discovered that Auxilin is required similarly for Delta internalization and signaling.
Why is ligand endocytosis by signaling cells required for signaling?
Many models have been proposed and they fall into two general classes: “pulling” models, or “recycling” models.
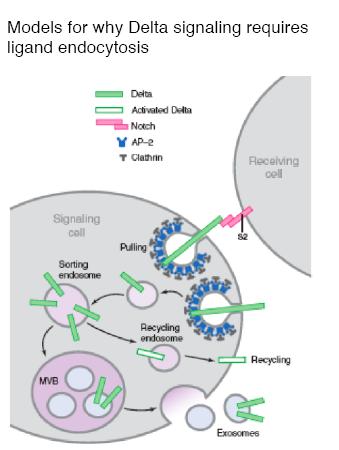 In “pulling” models, ligand is internalized after binding to the Notch receptor; ligand endocytosis generates mechanical forces that result in cleavage of the Notch intracellular domain (Notch activation), either by exposing the proteolytic cleavage site on the Notch extracellular domain, or by causing the heterodimeric Notch receptor to dissociate. Either of these events would lead to Notch receptor activation, that is, cleavage of the intracellular domain so that it can enter the nucleus. In “recycling” models, ligand internalization prior to receptor binding is required to process the ligand endosomally, and recycle it back to the plasma membrane in an activated form. The activated form could be a processed Delta, or Delta in exosomes. There is evidence for and against each of these ideas. For example, Delta and the Notch extracellular domain sometimes can be observed to colocalize in endosomes, supporting the idea that Delta internalization occurs after receptor binding to facilitate S2 cleavage. The e ndosome model may explain the longrange activity of Delta that is sometimes observed. The recycling model was first proposed as a result of an observation on the function of Epsin. In the wing and eye discs, most of the endosomal Delta observed is internalized in a Epsin independent manner. This led to the idea that Epsin-dependent internalization of Delta routes it to a specific endosomal pathway for activation and recycling. The idea is supported by an experiment in which replacement of the Delta intracellular domain with an LDL receptor fragment that mediates recycling results in Epsin-independent Delta signaling in the wing disc. Experiments with bristle cell precursors (sensory organ precursor cells or SOPs) also support a recycling model. Rab11-containing recycling endosomes are able to form only in the SOP neural precursor cell called pIIb, which may contribute to it becoming the Delta signaler. It is possible to imagine a model that involves both pulling and recycling; pulling of Delta bound to rec eptor could activate Notch initially, and then the Notch extracellular domain could be degraded in endosomes and a Delta receptor recycled to the plasma membrane.
In “pulling” models, ligand is internalized after binding to the Notch receptor; ligand endocytosis generates mechanical forces that result in cleavage of the Notch intracellular domain (Notch activation), either by exposing the proteolytic cleavage site on the Notch extracellular domain, or by causing the heterodimeric Notch receptor to dissociate. Either of these events would lead to Notch receptor activation, that is, cleavage of the intracellular domain so that it can enter the nucleus. In “recycling” models, ligand internalization prior to receptor binding is required to process the ligand endosomally, and recycle it back to the plasma membrane in an activated form. The activated form could be a processed Delta, or Delta in exosomes. There is evidence for and against each of these ideas. For example, Delta and the Notch extracellular domain sometimes can be observed to colocalize in endosomes, supporting the idea that Delta internalization occurs after receptor binding to facilitate S2 cleavage. The e ndosome model may explain the longrange activity of Delta that is sometimes observed. The recycling model was first proposed as a result of an observation on the function of Epsin. In the wing and eye discs, most of the endosomal Delta observed is internalized in a Epsin independent manner. This led to the idea that Epsin-dependent internalization of Delta routes it to a specific endosomal pathway for activation and recycling. The idea is supported by an experiment in which replacement of the Delta intracellular domain with an LDL receptor fragment that mediates recycling results in Epsin-independent Delta signaling in the wing disc. Experiments with bristle cell precursors (sensory organ precursor cells or SOPs) also support a recycling model. Rab11-containing recycling endosomes are able to form only in the SOP neural precursor cell called pIIb, which may contribute to it becoming the Delta signaler. It is possible to imagine a model that involves both pulling and recycling; pulling of Delta bound to rec eptor could activate Notch initially, and then the Notch extracellular domain could be degraded in endosomes and a Delta receptor recycled to the plasma membrane.
What are the roles of Clathrin and Epsin in ligand endocytosis?
There is also controversy regarding the role of Clathrin in Epsin-dependent endocytosis. Our genetic data indicate clearly that Delta internalization and signaling require clathrin; Clathrin heavy chain mutations are strong dominant enhancers of the liquid facets (lqf) mutant phenotypes. In addition, our more recent experiments with auxilin mutants lend strong support to the idea that clathrin is required for Delta signaling. Of course, this does not necessarily mean that Clathrin is being used to form CCVs. In fact, the results of studies in vertebrate cells suggest that proteins that use Ub as an internalization signal are endocytosed via a Clathrin-independent caveolar pathway, which nonetheless requires Epsin. Moreover, it was shown that Epsin cannot bind Ub and Clathrin simultaneously, and that Clathrin negatively regulates the association of Epsin with ubiquitinated cargo. More recent results of biochemical experiments are directly at odds with these studies. Epsin was shown clearly to associate with CCVs and not with caveolae. How Clathrin and Ub binding regulate Epsin in the context of Delta internalization is a critical issue that merits further experiments.
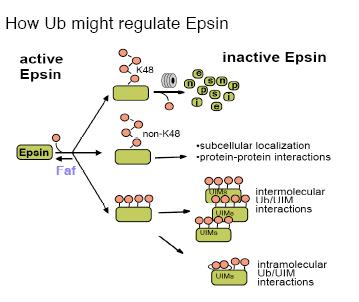 Regulation of Epsin by Ub.
Our discovery that Epsin is the critical substrate of the Fat facets (Faf) deubiquitinating enzyme in the Drosophila eye was the first demonstration that Epsin is regulated by Ub. Subsequently, experiments in vertebrate cell culture show that vertebrate Epsin is also ubiquitinated and deubiquitinated by Usp9, the human Faf homolog. Our genetic data show clearly that deubiquitination positively regulates Epsin activity; the faf and lqf mutant phenotypes are similar, and lqf mutants were identified as dominant enhancers of weak faf phenotypes. These genetic interactions indicate that faf+ and lqf+ work synergistically, and thus ubiquitination inactivates Epsin. In addition, our genetic and biochemical data indicate that Epsin is multi-ubiquitinated, and that ubiquitination might target Epsin for proteasomal degradation. By contrast, the data in vertebrate cells best fit a model where Epsin is mono-ubiquitinated, and in a UIM-dependent manner. Moreover, it was proposed that, perhaps through intramolecular int eractions between Ub and the UIMs, Ub would block Epsin function. How ubiquitination regulates Epsin function in Delta internalization is an important point that needs to be resolved.
Regulation of Epsin by Ub.
Our discovery that Epsin is the critical substrate of the Fat facets (Faf) deubiquitinating enzyme in the Drosophila eye was the first demonstration that Epsin is regulated by Ub. Subsequently, experiments in vertebrate cell culture show that vertebrate Epsin is also ubiquitinated and deubiquitinated by Usp9, the human Faf homolog. Our genetic data show clearly that deubiquitination positively regulates Epsin activity; the faf and lqf mutant phenotypes are similar, and lqf mutants were identified as dominant enhancers of weak faf phenotypes. These genetic interactions indicate that faf+ and lqf+ work synergistically, and thus ubiquitination inactivates Epsin. In addition, our genetic and biochemical data indicate that Epsin is multi-ubiquitinated, and that ubiquitination might target Epsin for proteasomal degradation. By contrast, the data in vertebrate cells best fit a model where Epsin is mono-ubiquitinated, and in a UIM-dependent manner. Moreover, it was proposed that, perhaps through intramolecular int eractions between Ub and the UIMs, Ub would block Epsin function. How ubiquitination regulates Epsin function in Delta internalization is an important point that needs to be resolved.
What experiments are we doing?
1. Determine which modules of Epsin are required for Delta signaling.
We are generating a large number of genomic DNA transgenes containing liquid facets genes that express proteins with different motifs deleted. We are testing the transgenes for their ability to support Delta signaling, Delta endocytosis, Epsin ubiquitination, and normal levels of Epsin accumulation.
2. Determine why Auxilin is required for Delta signaling.
We are performing transgene experiments to ask if Auxilin is required solely to produce free clathrin and/or Epsin. In addition, we are performing a large mutant screen for genes that interact with auxilin.
3. Determine how ubiquitination regulates Epsin.
We want to understand the relevance to Delta signaling of Epsin ubiquitination aund deubiquitination by Fat facets. We want to identify the E3 ubiquitin ligase that ubiquitinates Epsin and to use it in both genetic and biochemical experiments. We want to to map the ubiquitination sites on Epsin and characterize the types of ubiquitin chains attached to the protein. In addition, we want to characterize the mutant phenotype of the E3 mutants, to determine the developmental consequences when Epsin fails to be ubiquitinated. We know that Delta signaling fails in fat facets mutants, when Epsin is not deubiquitinated. But why is Epsin ubiquitinated in the first place?
4. Characterize the interactions between Epsin and the Ral GTPase.
We found Ral mutants as dominant enhancers of the rough eye caused by Epsin overexpression in the eye. It appears that Ral regulates Notch signaling through Epsin, and we are characterizing their interaction genetically and biochemically.
5. Characterize enhancers of liquid facets hypomorphs.
We performed a large mutant screen for modifiers of the rough eyes of liquid facets hypomorphs, and we identified nine lethal complementation groups as enhancers. We are identifying all of these genes, many or all of which are likely to participate in Notch signaling.
|
Lab News
- Stephen Fleenor is on his way to graduate school at Oxford!
- Markay Isaac is on her way to Vet School at A&M!
The Section of Molecular Cell and Developmental Biology is one of four units in the School of Biological Sciences. Other Sections are Integrative Biology, Molecular Genetics and Microbiology and Neurobiology.
|